We know from experiments that these lines are unique to specific
atoms, but how are the lines created, and why does each atom
have its own unique fingerprint?
Birth of Quantum Mechanics in the early 20th century:
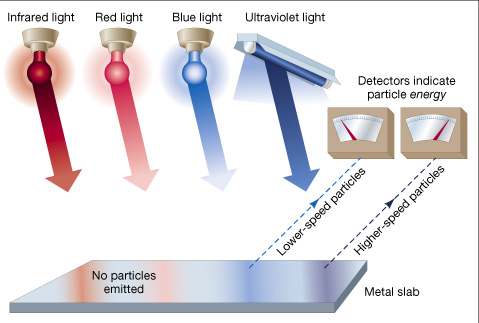
- Light can behave as a particle with discrete energy (Einstein 1905).
Photon Energy = Planck's constant X frequency
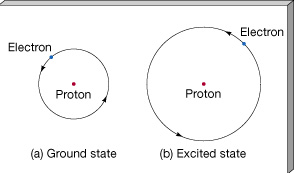
- Electrons in an atom can exist only with specific values of energy (Bohr 1912).